Global news
AFRICA, ANGE KASONGO, ASIA, CLINICAL TRIALS, CONGO, CONGO (KINSHASA), DEMOCRATIC REPUBLIC OF CONGO, DISEASE OUTBREAKS, EUROPE, IFPMA, INTERNATIONAL FEDERATION OF PHARMACEUTICAL MANUFACTURERS AND ASSOCIATIONS, JAPAN, JENNIFER RIGBY, KINSHASA, KM BIOLOGICS, LONDON, PANDEMIC, PAULA BARBOSA, PUBLIC HEALTH, REUTERS, SAMUEL ROGER KAMBA MULAMBA, SONIA ROLLEY, UNITED KINGDOM
Fatima Khan
0 Comments
Challenges in Mpox Vaccination Rollout for Children in Congo Due to Legal Hurdles
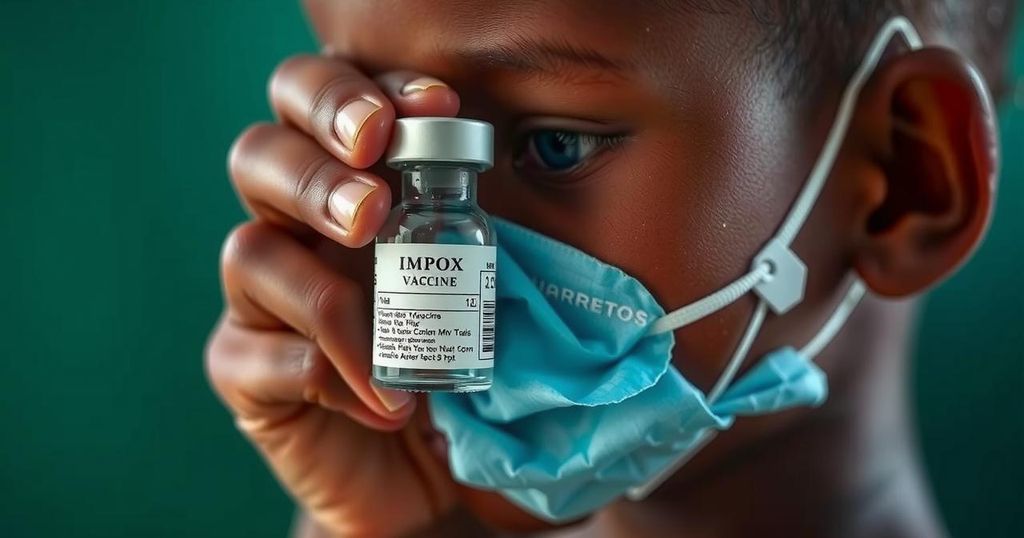
Vaccinations against mpox have started for adults in Congo, but children, the most at risk, do not have access due to legal complications regarding liability for potential side effects of the vaccine donated by Japan. After negotiations, Congo’s government claims the issue is resolved, emphasizing the urgent need for better global health systems to facilitate timely vaccination efforts.
In the Democratic Republic of Congo, vaccinations against mpox for adults have commenced; however, children, who are particularly vulnerable to the disease, are left without available doses due to a protracted legal dispute. Japan had promised to contribute three million doses of the LC16m8 vaccine, specifically approved for pediatric use, but negotiations regarding liability for potential side effects have caused delays. The Congolese government has since stated that the issue has been resolved, highlighting the critical need for improved systems to manage vaccine-related liabilities to facilitate timely public health responses.
Following Japan’s pledge in September, discussions centered on liability coverage have revealed a persistent challenge in global health responses, wherein poorer nations fear financial repercussions from any adverse vaccine effects. The urgency remains high, as children have acquired a significant share of the over 1,100 mpox fatalities reported in Africa this year. Health experts argue for the implementation of efficient compensation systems, drawing comparisons to similar complications seen during the COVID-19 pandemic.
The LC16m8 vaccine received emergency use authorization from the World Health Organization and is administered using a specialized bifurcated needle; however, adequate training for health personnel is necessary for its delivery. Ongoing vaccine distribution is also hampered by insufficient public awareness in affected communities. Amidst these challenges, Japan’s government is actively working to resolve outstanding issues related to the vaccine donations.
As the government of Congo finalizes formalities for the importation of the needed pediatric vaccine, it emphasizes the importance of expediting its arrival while ensuring that practical frameworks are in place for effective vaccine administration, thus protecting the lives of the most vulnerable individuals in the society.
The article discusses the challenges faced in rolling out mpox vaccinations for children in the Democratic Republic of Congo amid legal and logistical hurdles. Japan had pledged a substantial donation to combat mpox, but negotiations regarding liability issues delayed the vaccine’s distribution. This situation illustrates a broader concern within global health initiatives about ensuring that countries can safely implement health interventions without incurring exorbitant liabilities. The importance of timely interventions to protect vulnerable populations, especially children, against dire health threats like mpox is underscored.
In summary, the delay in providing mpox vaccinations for children in the Democratic Republic of Congo highlights critical issues surrounding liability in vaccine distribution. The resolution between Japan and Congo regarding responsibility for side effects demonstrates the need for improved international frameworks to facilitate the rapid administration of vaccines during health crises. The ongoing public health challenge necessitates swift action to ensure that vulnerable populations receive the necessary protections without delay.
Original Source: www.usnews.com



Post Comment